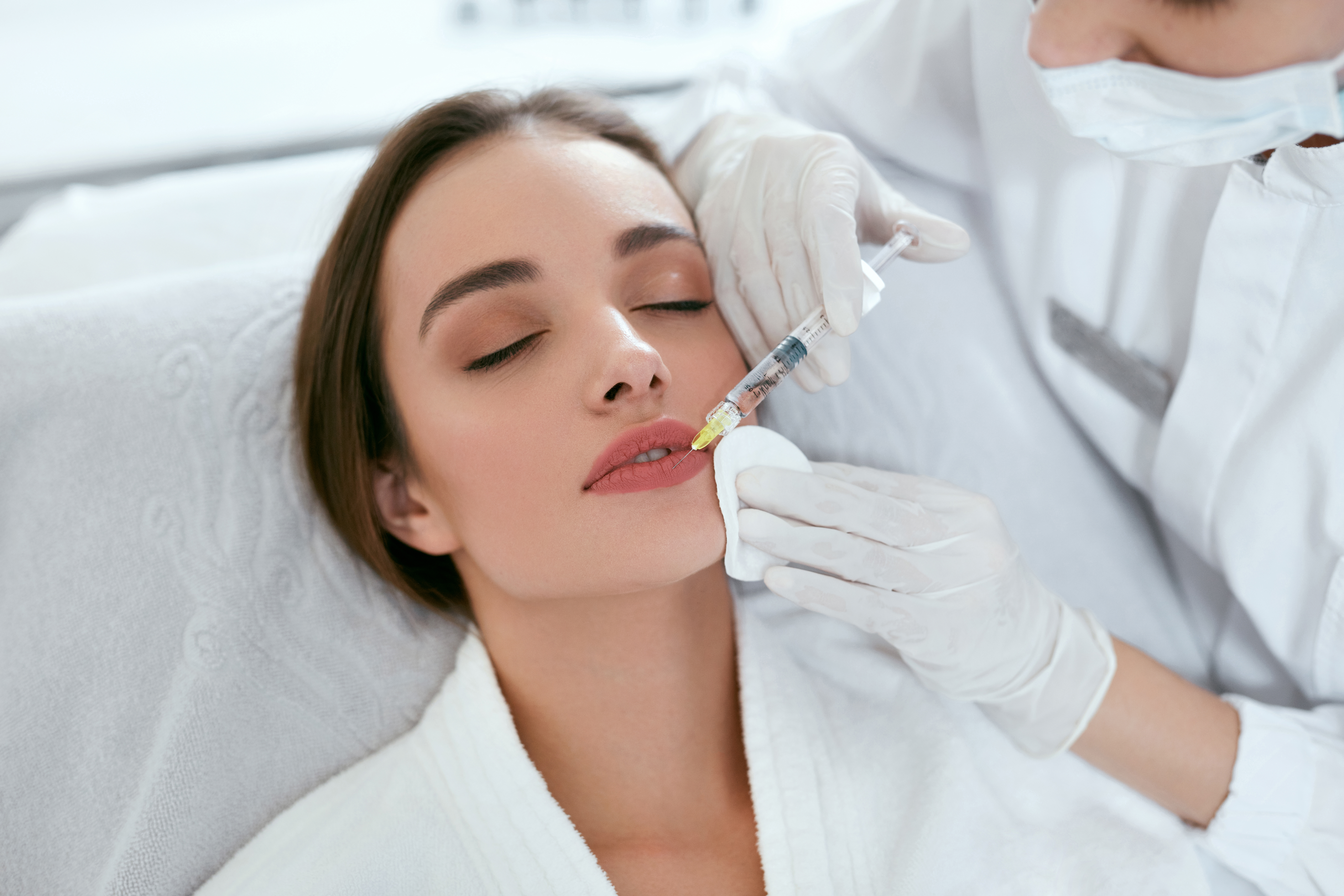
Botox for Crow’s Feet Now Approved by the FDA
Conveniently located to serve the areas of Maryland, Virginia and Washington, D.C.
Posted on January 19, 2017 under Medical Spa
Cosmetic Surgery Associates is a premier practice based out of McLean, VA, and Bethesda, MD, providing a range of plastic surgery and other cosmetic procedures. For plastic surgery in Virginia and Maryland, Cosmetic Surgery Associates, led by board-certified plastic surgeons, Dr. Franklin Richards and Dr. Dean Jabs, provides safe, proven, and effective treatments.
However, while cosmetic surgery in Maryland and Virginia from Dr. Richards and Dr. Jabs is an excellent option for many patients, some of them may need only non-surgical treatments such as Botox. The prolific and exciting news for such patients looking for Botox treatment is that the FDA has now approved this treatment for the reduction of crow’s feet. The treatment was already approved for the reduction of frown lines between the brows, and now the scope of the cosmetic benefits of Botox has been increased to include crow’s feet treatment.
Botox Benefits
Unlike plastic surgery in Maryland or Virginia at Cosmetic Surgery Associates, Botox does not offer long-lasting benefits, and the effects may last for anywhere between three and six months. However, it is a simpler and faster procedure involving tiny injections of botulinum toxin in a highly diluted form, which are injected directly into the facial muscles that are causing frown lines, crow’s feet, or other lines and wrinkles. Botox paralyzes the targeted muscles, which results in the fading away of the wrinkle for a few months.
Botox is not just useful for the cosmetic treatment of frown lines and crow’s feet, but it also has a number of medical applications. The FDA has approved Botox also for the medical treatment of conditions such as eyelid spasms, heavy perspiration under the arms, and acute migraines. Unlike cosmetic surgery in Virginia, Maryland, or other places, a Botox treatment does not involve any significant downtime, which makes it an ideal choice for busy people who are looking for temporary relief from facial wrinkles.
Approval for Crow’s Feet Treatment
Crow’s feet are clinically known as lateral canthal lines, which are believed to be caused by the repetitive action of eye muscles over a prolonged time period. Repetitive facial expressions such as frowning, smiling, or squinting may cause the affected muscles to shrink over a period of time, resulting in a permanent wrinkle. These fine lines called crow’s feet can be reduced temporarily with Botox. Eye conditions such as eyelid twitching or blepharospasm were already being treated with Botox, and now cosmetic conditions such as crow’s feet can also be treated using the same treatment principle.
According to the FDA’s official statement with regard to the approval of Botox for crow’s feet treatment, Botox is a temporary but effective treatment to reduce wrinkles that may appear near aging eyes. When these wrinkles or crow’s feet are reduced with the help of Botox, the face becomes smoother and the eyes look more refreshed and rejuvenated. It can take away the old and tired look from a person’s eyes. Since Botox is an injectable treatment with almost instant benefits, it has been among the most popular non-surgical treatments around the world.
Clinical Study
Botox received FDA’s approval for cosmetic treatment of crow’s feet only after the drug’s manufacturer Allergan, Inc. of Irvine, CA was able to show the successful results of the treatment in a study of 833 adults. The group of 833 participants was assigned randomly to receive a placebo injection of Botox, while another group was observed without this treatment. The group that was treated with Botox revealed a significant reduction in the appearance of crow’s feet around the eyes, compared to the other group that was not treated.
The treatment has not been approved by the FDA, but experienced surgeons advise their patients not to have similar expectations from Botox as they would have from cosmetic surgery in Maryland or Virginia. Botox can be used only in specific areas of the face as approved by the FDA. The results are temporary, and Botox is not suitable to treat deep wrinkles, creases, or folds on the face. Patients who are fully aware of the pros and cons of Botox are likely to achieve better satisfaction levels because they can make an informed decision and have realistic expectations of the outcome.
Precautions
The FDA warning label on Botox says that the toxin’s impact may spread to other parts of the body, which may cause symptoms that are similar to botulism. However, it also notes that a confirmed case of such an event taking place has been known when Botox is used in prescribed doses. Therefore, the key to safety with Botox is to receive treatment from a qualified and experienced Botox provider.
Source of News: FDA Press Release
To learn more about Breast Augmentation procedures performed by this practice please visit: http://breastaugmentationmd.com/
Our Plastic Surgery Associates team includes Dr. Franklin Richards, Dr. A. Dean Jabs, and Dr. Keshav Magge. Each of our plastic surgeons is board-certified, and together they have over 60 years of combined experience. Drs. Richards, Jabs, and Magge are all highly qualified in procedures for the face, breast, and body, and pride themselves in providing excellent results through our state-of-the-art, Quad A certified operating centers